Understanding the Boots Paracetamol Recall
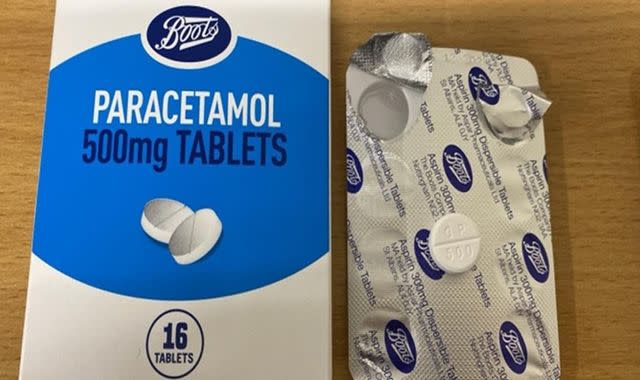
Introduction to the Recall
The Boots paracetamol recall has raised significant concerns among consumers and health professionals alike. Paracetamol is a commonly used pain reliever, and any issues with its safety can have serious implications for public health. The recent recall, initiated by Boots UK, has become a pressing topic within the pharmaceutical and retail sectors, prompting discussions about quality control measures and consumer safety.
Details of the Recall
On October 5, 2023, Boots UK announced a voluntary recall of specific batches of paracetamol due to concerns over potential contamination. The affected products include various formulations of paracetamol sold under the Boots brand name, including both standard and extra strength variants. The company stated that the decision was made as a precautionary measure after reports of unexpected contaminants were identified during routine quality checks.
Impact on Consumers
This recall affects a wide range of products that have been sold in Boots stores nationwide and online. Customers who have purchased affected paracetamol products are urged to check the batch numbers and expiry dates. Boots has advised consumers to return any affected products to their nearest store for a refund. The recall has stirred concerns, particularly among those who rely on paracetamol for pain management and fever relief.
Regulatory Response
The Medicines and Healthcare products Regulatory Agency (MHRA) is closely monitoring the situation. According to a spokesperson for the MHRA, the safety of pharmaceutical products is paramount, and Boots is cooperating fully with the investigation. They reassured the public that the risk of adverse health effects from the recalled products is being assessed, and guidelines for safe use are being communicated effectively.
Conclusion and Future Considerations
This incident emphasizes the importance of vigilance in the pharmaceutical industry, particularly with widely-used medications like paracetamol. Consumers are encouraged to stay informed about product recalls and to be proactive in seeking information. As Boots rectifies the situation, it will likely spark more stringent regulations and checks on over-the-counter medications in the future. The long-term implications of this recall remain to be seen, but it serves as a vital reminder of the need for quality assurance in pharmaceuticals.